Aluminum and Carbon Fiber are both renown for providing lightweight strength and high-performance mechanical properties to components and assemblies. These materials are most often used in combination in the aerospace and auto racing industries. Alone Carbon Fiber laminates and Aluminum are both highly resistant to any form of corrosion. But when the materials are paired together on mating surfaces having physical contact, galvanic corrosion can quickly affect those surfaces and later cause accelerated corrosion most commonly in the aluminum but can also degrade the carbon fiber composites.
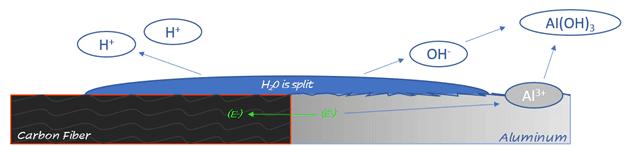
Galvanic corrosion occurs when dissimilar metals are met with electrical contact in the presence of an electrolyte, most commonly water. Aluminum and Carbon Fiber are both conductors of electricity but have much different electrical potentials. Carbon Fiber actively participates in the galvanic corrosion process of aluminum by an oxygen reduction or water splitting reaction. Because carbon fiber is the more noble of the materials, the aluminum gives away electrons and is then corroded over time.
To combat Galvanic Corrosion, one may consider just taking water out of the equation. This seems practical enough until one considers that the oxygen we breathe has a relative moisture content in it, known as humidity. This reaction with relative humidity is much slower than if the carbon fiber and aluminum are constantly exposed to water but galvanic corrosion may still occur. If the life cycle for an aluminum and carbon fiber assembly are relatively short, less than a month, galvanic corrosion should not affect the assembly enough to cause premature failure of the assembly. It is however critical to know how the assembly will be exposed to humidity conditions and the amount of surface area the two parts share directly in comparison to the total area of the part.
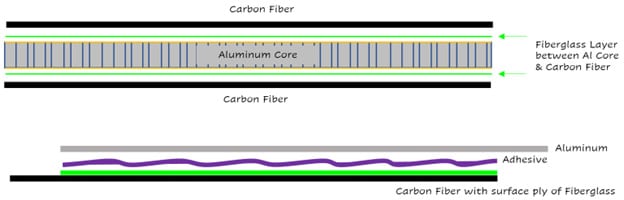
The most common solution to preventing corrosion between the two materials involves creating a physical barrier or isolation point between the two conductive materials. This can be achieved a few different ways. As previously discussed, Fiberglass does not carry conductive properties, making it a great choice blocking the mechanism of the corrosion. Fiberglass can be added to the layup where aluminum core meets core fiber. (As shown below) or where CF & Aluminum substrates may be bonded together.